OWLiver® Overview
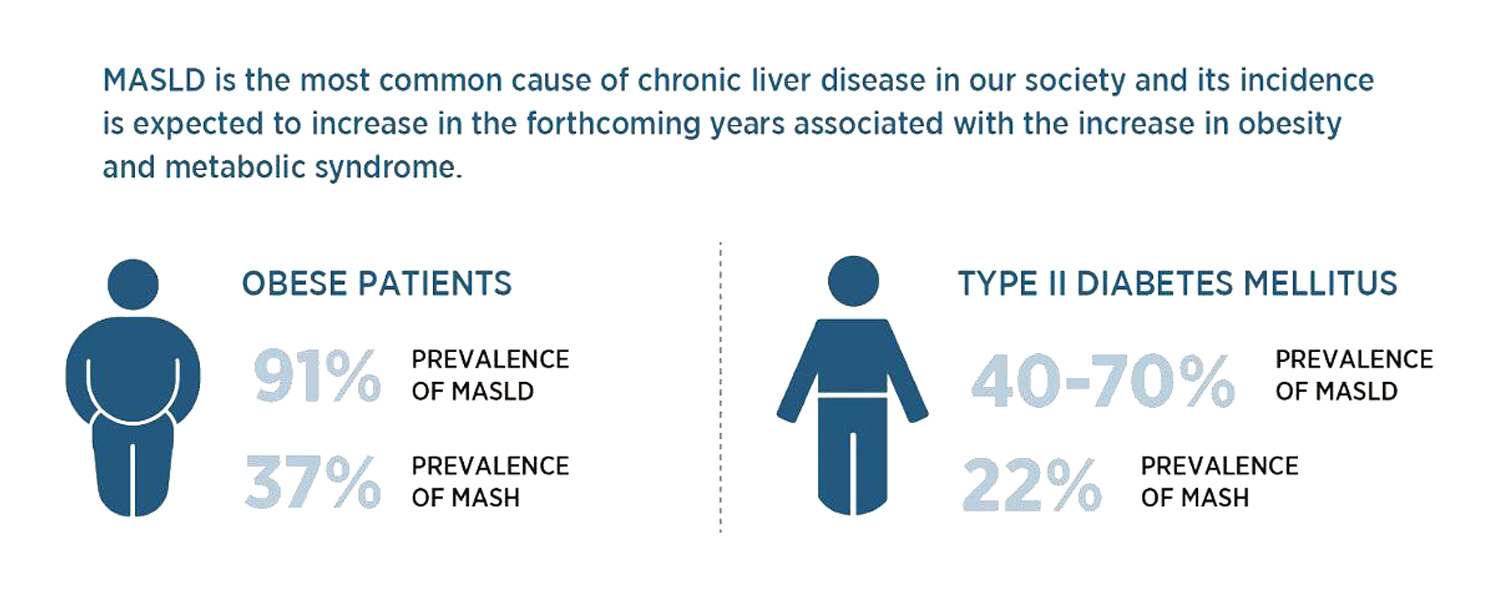
OWLiver® is a non-invasive blood test to assess MASLD. It analyzes a panel of biomarkers through metabolomics methodology. All metabolites are measured by high performance liquid chromatography and mass spectrometry (UHPLC-MS) 1,2. The analysis of these metabolites allow assessment of the amount of fat, inflammation and fiber in the liver3-6. OWLiver® makes it possible to accurately establish the degree of progression of Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD; known before as non-alcoholic fatty liver disease NAFLD, or MALFD. MASLD is the most common cause of chronic liver disease in our society. MASLD may not cause symptoms. Having obesity or Type 2 diabetes increases the risk of developing it. Altered lipid metabolism is also associated with MASLD progression; alterations in liver and serum lipidomic signatures are good indicators of disease development and progression5. Proactively identifying patients with a higher risk of disease progression, preventing unfavorable outcomes, and providing superior management that can reduce the mortality for all cases should be of top priority.
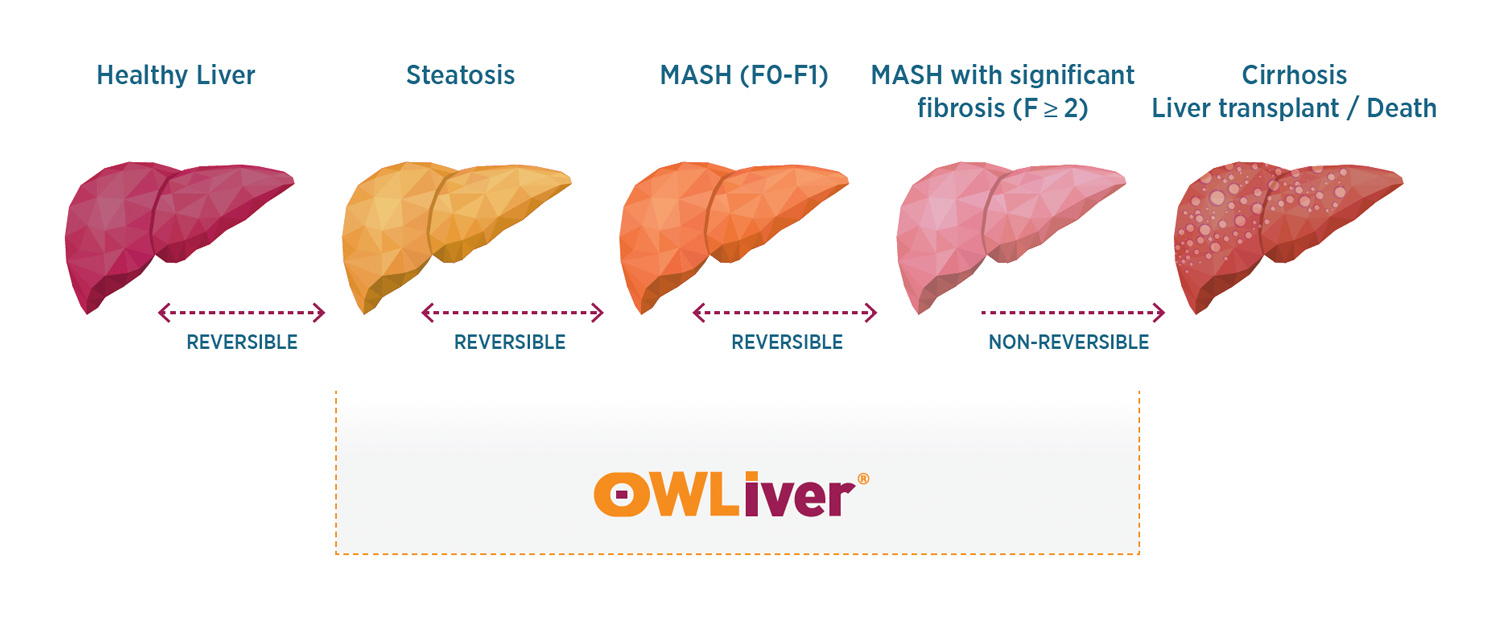
The OWLiver® test is a serum non-invasive test for detecting MASLD, MASH with moderate or no fibrosis F0-F1, and “at-risk” MASH (MASH with significant and advanced fibrosis F≥2-F3) based on metabolomics (Figure 23). The test has been validated vs. gold standard liver biopsy. The OWLiver® test consists of a fasting blood lipidomic analysis, allowing for the measurement of a panel of lipid biomarkers. These biomarkers reflect the amount of fat and inflammation in the liver and the degree of fibrosis of the liver, making it possible to establish the degree of development of metabolic dysfunction-associated steatotic liver disease (MASLD).
OWLiver® Intended Use
The OWLiver® test is a serum non-invasive test for detecting MASLD, MASH with moderate or no fibrosis F0-F1, and “at-risk” MASH (MASH with significant fibrosis F≥2) based on metabolomics (Figure 1). The test has been validated vs. gold standard liver biopsy. The OWLiver® test consists of a fasting blood lipidomic analysis, allowing for the measurement of a panel of lipid biomarkers. These biomarkers reflect the amount of fat and inflammation in the liver and the degree of fibrosis of the liver, making it possible to establish the degree of development of metabolic dysfunction-associated steatotic liver disease (MASLD).
OWLiver® is indicated for use in adult patients suspected of having MASLD, a condition that typically presents without symptoms. Suspicion of MASLD may arise from any combination of the following factors:
- Elevated transaminase levels in liver function tests
- Adult with a body mass index (BMI) >30 kg/m2
- The presence of risk factors such as obesity, diabetes, metabolic syndrome, dyslipidemia, or polycystic ovary syndrome
- FIB-4 > 1.3
Early identification is critical, as patients at risk of progressing to severe MASLD can significantly benefit from targeted therapeutic interventions. Before performing OWLiver®, it is necessary to rule out other possible causes of fatty liver. OWLiver® was not studied in the following patient populations and therefore considered outside intended use:
- Patients with liver disease induced by viruses or medication.
- Patients diagnosed with any other liver disease.
- Patients with increased alcohol consumption: more than 25 g/day in women and 40 g/ day in men.
- BMI <25 kg/m2
Because OWLiver® is a metabolomics-based test, the results are dynamic reflections of disease status and can be highly responsive to intervention. OWLiver® recommended testing frequency is very similar to the diabetes guidelines (ADA) regarding recommended lab test monitoring. Depending on a patient’s symptoms, history, intervention, and risk, clinician’s frequency of use of OWLiver® varies from every 6 months to one-year for monitoring effectiveness of clinical intervention and current risk levels.
Sample Extraction, Conservation, and Transport for OWLiver® Test
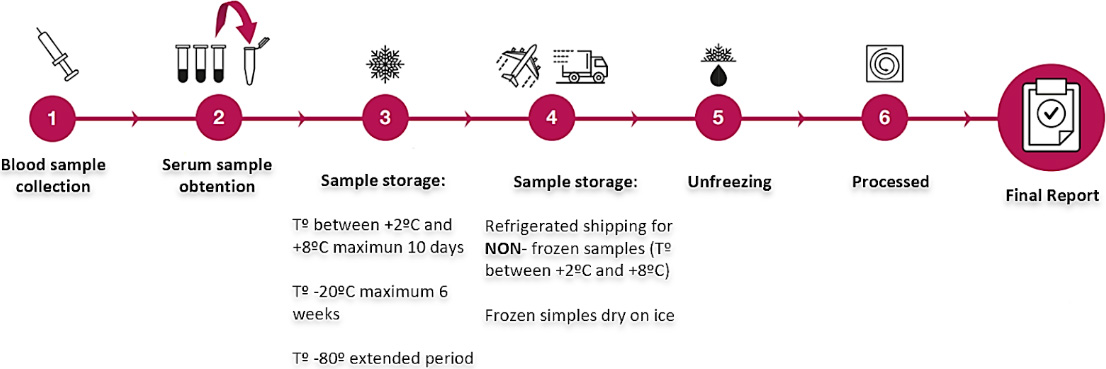
Figure 5: Simple sample collection and processing allows for broader utilization. Average turn-around times are 10-14 days.
OWLiver® Quick Facts:
Allows a reliable diagnosis of the patient in any harmful phases of NAFLD.3-6
Allows monitoring the follow-up of the patient, seeking surveillance in the regression or progression of NASH and liver fibrosis.3-6
References
- Barr J, Caballería J, Martínez-Arranz I, Domínguez-Díez A, Alonso C, Muntané J, et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J Proteome Res. 2012;11(4):2521 -32.2.
- Barr J, Vázquez-Chantada M, Alonso C, Pérez-Cormenzana M, Mayo R, Galán A, et al. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated. J Proteome Res. 2010;9(9):4501 -12.
- Mayo R, Crespo J, Martínez‐Arranz I, Banales JM, Arias M, Mincholé I, et al. Metabolomic‐based noninvasive serum test to diagnose nonalcoholic steatohepatitis: Results from discovery and validation cohorts. Hepatol Commun. 2018;2(7):807 -20.
- Bril F, Millán L, Kalavalapalli S, McPhaul MJ, Caulfield MP, Martinez-Arranz I, et al. Use of a metabolomic approach to non-invasively diagnose non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2018;20(7):1702 -9.
- Martínez‐Arranz I, Mayo R, Banales J, Mincholé I, Ortiz P, Bril F, et al. Non‐invasive serum lipidomic approach to discriminate non‐alcoholic steatohepatitis in multiethnic patients with type 2 diabetes mellitus. Hepatol. 2019; 70(1):1030A.
- Noureddin M, Mayo R, Martínez-Arranz I, Mincholé I, Cusi K, Bril F, et al. Serum-based Metabolomics Advanced Steatohepatitis Fibrosis Score (MASEF) for the non- invasive identification of patients with non-alcoholic steatohepatitis with significant fibrosis. J Hepatol. 2020; 73(1): S136.
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73(1):202-209
8. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions,risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.