Metabolic dysfunction-associated steatotic liver disease (MASLD) is rapidly increasing in prevalence, yet accurate and scalable tools for diagnosis and disease staging continue to pose challenges. Non-invasive tests (NITs) remain central to care pathways, but many face limitations—including the well-known sensitivity/specificity trade-off that complicates the detection of “at-risk metabolic dysfunction-associated steatohepatitis” (“at-risk MASH”). As clinicians aim to reduce unnecessary liver biopsies while still identifying patients with significant fibrosis, more reliable and complementary diagnostic strategies are needed.
The FIB-4 index is widely recommended as the first-line tool for fibrosis risk assessment due to its low cost and simplicity. However, its sizable indeterminate zone result leaves clinicians uncertain about next steps. The Metabolomics-Advanced Steatohepatitis Fibrosis Score (MASEF), part of the OWLiver® test, has shown strong performance in identifying at-risk MASH and offers potential as a second-line assessment. The OWLiver® test assesses steatosis, MASH, and at-risk MAH, providing greater visibility into the full path of liver health.
At the recent AASLD Liver Meeting, held in Washington, DC, on Nov 7-11, 2025, Dr. Mirella Zulueta from CIMA Sciences presented the poster: “Maximizing the Diagnostic Yield of Non-Invasive Tests: Simultaneous Optimization of Key Performance Metrics for Fibrotic Liver Disease with a FIB-4 and MASEF (OWLiver) Strategy.”
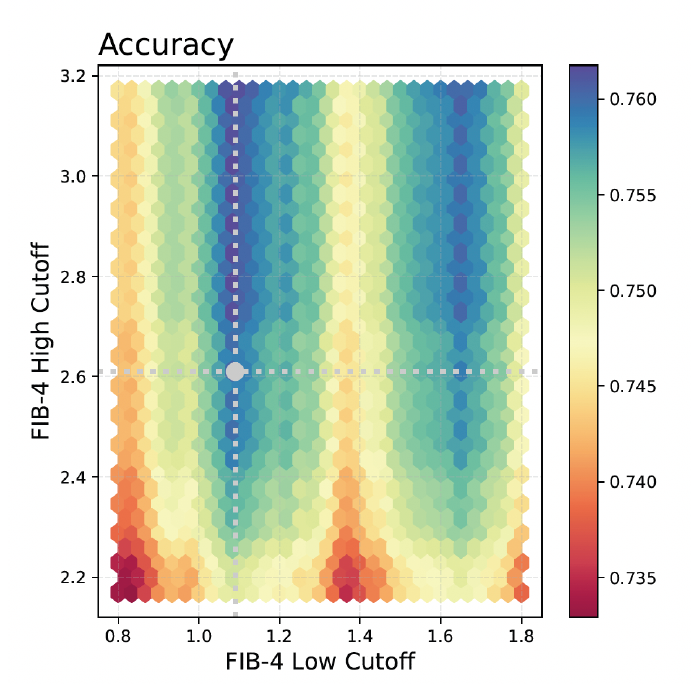
In a multicenter study of 1,122 adults with metabolic risk factors for MASLD, Martinez-Arranz et al. evaluated a tiered strategy that applied MASEF to patients whose FIB-4 values fell within the indeterminate range (FIB-4 index between 1.3 and 2.6). A systematic grid-based analysis of all possible FIB-4 cutoff pairs was performed, allowing researchers to assess how various threshold combinations influenced diagnostic performance of the FIB-4 + MASEF strategy.
This analysis led to optimized FIB-4 thresholds of 1.08 and 2.61, which balanced all five key diagnostic metrics for detecting at-risk MASH. When paired with a MASEF cutoff of 0.33, the combined approach achieved 78% accuracy, 71% sensitivity, 82% specificity, 64% PPV, and 88% NPV.
These findings show that simultaneous improvement across all major diagnostic metrics is achievable with a combined FIB-4 and MASEF strategy. This integrated pathway has the potential to streamline clinical decision-making, reduce uncertainty in intermediate-risk patients, and support more precise management of individuals with suspected MASLD.
